[ad_1]
Singapore-based medical tech Aevice Health has received undisclosed funding from venture capitalist East Ventures for its expansion into Southeast Asia.
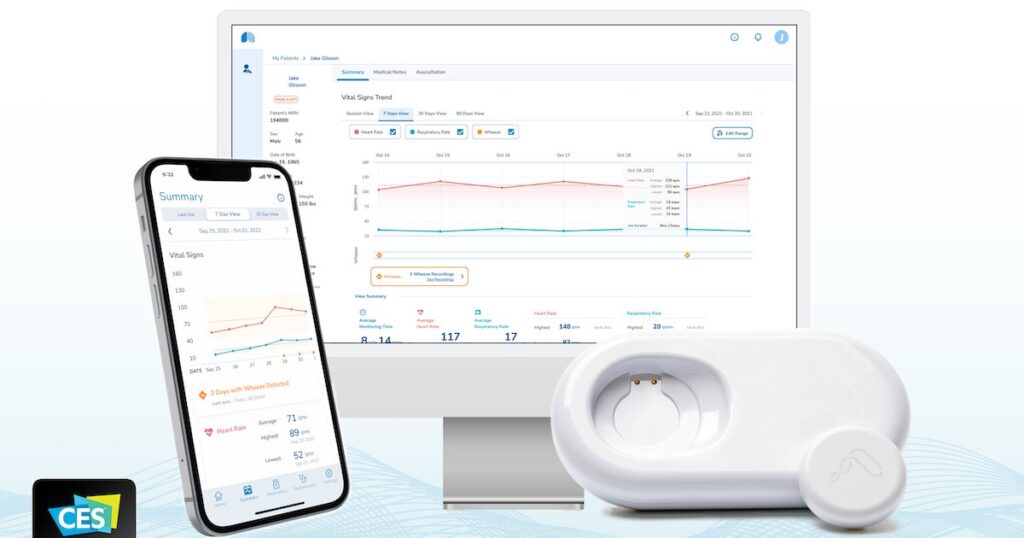
It also announced that its flagship product, the AeviceMD Smart Wearable Stethoscope, has received 510(k) clearance from the United States Food and Drug Administration.
AeviceMD is part of the AeviceMD Surveillance System, a patient management platform that collects and then analyzes lung sounds for signs of respiratory exacerbation.
WHY IS IT IMPORTANT
According to Aevice Health CEO Adrian Ang, chronic respiratory diseases in Southeast Asia represent a “significant untapped opportunity”. More than 7% of the population, or approximately 48.5 million people, suffer from chronic respiratory diseases, including COPD and asthma. The company is looking to expand its solution across the region to help solve this growing problem.
Meanwhile, its latest US FDA approval also enables the company to expand access to its respiratory care solution to more than 41 million US patients with COPD and asthma. “With this clearance, we are taking a big step towards becoming the equivalent of continuous blood glucose monitors for diabetes, but for respiratory health – a patient-centric, affordable and accessible solution that enables patients to recover healthily from the comfort of their home,” said Ang.
THE WIDER CONTEXT
These back-to-back announcements follow the Health Sciences Authority of Singapore’s approval for the AeviceMD monitoring system in March.
Two years ago, the company raised about $2 million and secured a local partnership to scale the Aevice® stethoscope in Japan.
Aevice Health is now working with Cedars-Sinai Medical Center to pilot its respiratory monitoring system remotely. It also plans to have its proprietary lung sound analysis algorithm approved by the US FDA “in the near future.”
[ad_2]
Source link